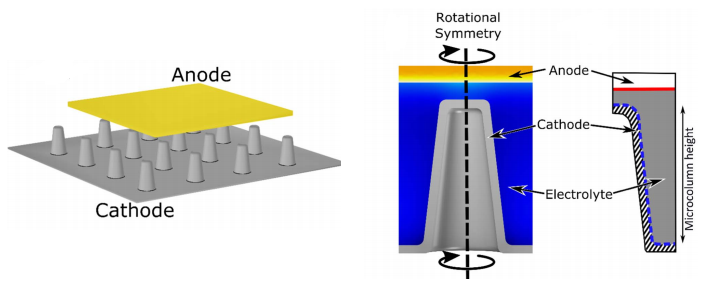
Transport in Thin Layer Batteries
The need for smaller, more powerful, higher capacity batteries is ever increasing. This has resulted in the development of ultra-thin (ca. 10 nm) batteries in which the anode and cathode are separated by an ultra-thin electrolyte layer. At this length scale electrical double layers, that form at the electrodes (both the anode and cathode), begin to overlap and result in changes to the molecular transport of Li ions. We investigate this phenomenon theoretically using finite element simulations and experimentally using ultra-thin electrochemical cells.
Select Publications:
Microscale 2.5D Batteries
K. McKelvey, A. A. Talin, B. Dunn, and H. S. White
Journal of the Electrochemical Society, 2017, 164(12), A2500-A2503.
Fabrication, Testing and Simulation of All Solid State Three Dimensional Li-ion Batteries
A. A. Talin, D. Ruzmetov, A. Kolmakov, K. McKelvey, N. Ware, F. El Gabaly, B. S. Dunn,
and H. S. White
ACS Appl. Mater. Interfaces, 2016, 8(47), 32385-32391.
Redox Cycling in Nanogap Electrochemical Cells. The Role of Electrostatics in Determining
the Cell Response
Q. Chen, K. McKelvey, M. A. Edwards, and H. S. White
J. Phys. Chem. C, 2016, 120(31), 17251–17260.
Ion Transport within High Electric Fields in Nanogap Electrochemical Cells
J. Xiong, Q. Chen, M. A. Edwards, and H. S. White
ACS Nano., 2015, 9(8), 8520-8529.
Electron-Transfer Kinetics and Electric Double Layer Effects in Nanometer-Wide Thin-Layer
Cells
L. Fan, Y. Liu, J. Xiong, H. S. White, and S. Chen
ACS Nano., 2014, 8(10), 10426-10436.