Synthetic Organic Electrochemistry
Electrochemistry represents a powerful tool within synthetic organic chemistry. Electrodes can be used to generate highly reactive and short-lived species, which are otherwise unfeasible to create/use in synthetic organic chemistry. Moreover, the current produced at these electrodes can be used to monitor the presence of electroactive species and probing reaction mechanisms.
As a member of the NSF funded Center for Chemical Innovation: Center for Synthetic Organic Electrochemistry, the White group is at the forefront of developing a fundamental understanding of green, safe, and economic electrochemical reactions. In doing this, we employ a range of electrochemical techniques, e.g., voltammetry, scanning electrochemical microscopy (SECM), enabling the characterization of kinetically fast reactions over short time-scales.
References:
Investigation of the Electrocatalytic Reduction of Peroxydisulfate Using Scanning
Electrochemical Microscopy
Hosseini, S., Solymosi, G.T. and White, H.S.,
Anal. Chem.2024, 96(21), 8424–8431
Unraveling hydrogen atom transfer mechanisms with voltammetry: oxidative formation
and reactivity of cobalt hydride
Boucher, D.G., Pendergast, A.D., Wu, X., Nguyen, Z.A., Jadhav, R.G., Lin, S., White,
H.S. and Minteer, S.D.,
Journal of the American Chemical Society, 2023, 145(32), 17665-17677.
Electroorganic synthesis in aqueous solution via generation of strongly oxidizing
and reducing intermediates
Hosseini, S., Beeler, J.A., Sanford, M.S. and White, H.S.,
Faraday Discussions, 2023, 247, 192-205.
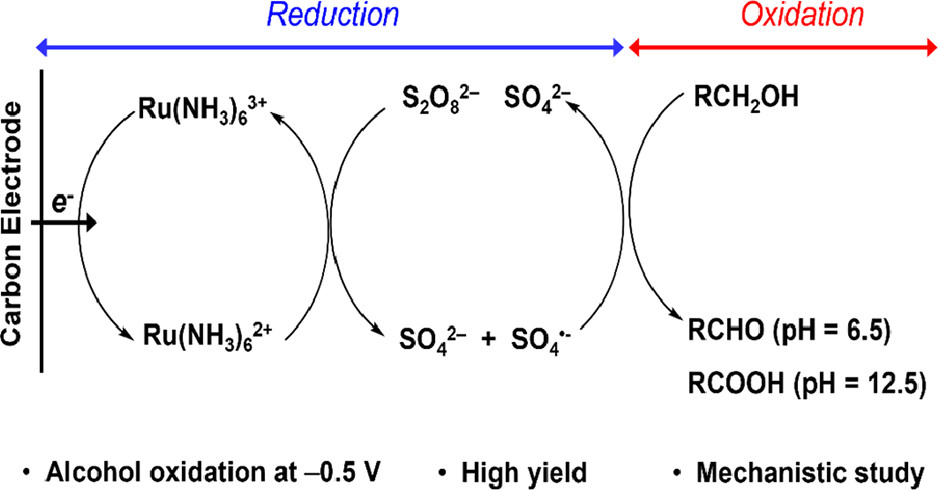
Oxidation by Reduction: Efficient and Selective Oxidation of Alcohols by the Electrocatalytic
Reduction of Peroxydisulfate
S. Hosseini, J. N. Janusz, M. Tanwar, A. D. Pendergast, M. Neurock, and H. S. White
J. Am. Chem. Soc. 2022, 144(46), 21103–21115
Electrochemical Reduction of [Ni(Mebpy)3]2+. Elucidation of the Redox Mechanism by
Cyclic Voltammetry and Steady-State Voltammetry in Low Ionic Strength Solutions.
ChemElectroChem. 2020, 7(6), 1473-1479
Electrochemically Driven, Ni-Catalyzed Aryl Amination: Scope, Mechanism, and Applications
Y. Kawamata, J. C. Vantourout, D. P. Hickey, P. Bai, L. Chen, Q. Hou, W. Qiao, K.
Barman, M. A. Edwards, A. F. Garrido-Castro, J. N. deGruyter, H. Nakamura, K. W. Knouse,
C. Qin, K. J. Clay, D. Bao, C. Li, J. T. Starr, C. Garcia-Irizarry, N. Sach, H. S.
White, M. Neurock, S. D. Minteer, and P. S. Baran
J. Am. Chem. Soc., 2019, 141(15), 6392-6402.
