Scott L. Anderson Research Group

Contact Information
Office: 1224 Henry Eyring Building (HEB) North wing
Department of Chemistry, University of Utah,
315 S. 1400 E. RM Dock, Salt Lake City, UT 84112-0850
Phone : (801) 585 - 7289 FAX: (801)581-8433 Group: (801) 581-6644
E-Mail : anderson@chem.utah.edu
ORCID 0000-0001-9985-8178
How to Find the Henry Eyring Building
Education
B. A. Rice University, 1977 (P. R. Brooks)
Ph. D. University of California at Berkeley, 1981 (Y. T. Lee)
Postdoctoral, Stanford University, 1981-83 (R. N. Zare)
Awards
ACS Physical Division Award in Experimental Physical Chemistry 2016
Chair, Div. Chemical Physics, American Physical Society 2018-2019
Robert W. Parry Teaching Award (2015)
Distinguished Professor, U of Utah, 2011
Fellow, American Assoc. for the Advancement of Science, 2011
U of Utah Distinguished and Creative Research Award, 2007
Fellow, American Physical Society, 2005
NSF Creativity Award, 2004-2006
Invitation Fellow, Japan Society for the Promotion of Science, 2002
Professeur Invité, Université Paris-Sud, 1990, 1991
Visiting Scientist, Fakultät für Physik der Universität Freiburg, 1990
Camille and Henry Dreyfus Foundation Teacher-Scholar, 1989-1994
Japan Society for the Promotion of Science Fellow, 1989-1990
Alfred P. Sloan Foundation Research Fellow,1988-1990
Research Interests – Physical and Analytical Chemistry
Anderson group research is generally in the area of nanoparticle surface chemistry, but with four distinct research foci, ranging from single nanoparticles isolated in the gas phase, to size-selected catalysts and electrocatalysts, to methods for production and characterization of surface-functionalized high energy density nanoparticles for propellant applications.
The title for each of the sections below is a link to more information, including recent results. Information about earlier projects in fullerene chemistry, state-selected ion chemistry, gas-phase metal cluster chemistry, and soft x-ray photochemistry can be found in the publication list linked to below. There are also links to several software and hardware projects that may be of interest.
Size-Selected Model Supported Catalysts
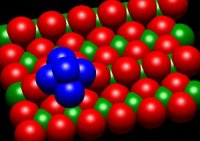 The goal of these experiments is to probe the correlations between supported cluster
size, morphology, electronic structure, adsorbate binding sites, support properties,
and catalytic activity, and thereby, to learn about catalyst active sites, mechanisms,
and approaches to improving activity/selectivity. We use size- and energy-selected
metal cluster ion deposition on well-characterized oxide surfaces to prepare model
catalysts, which are then studied using a variety of in situ techniques. The electronic
structure of the samples is probed by a combination of XPS, UPS, and INS. Low energy
ion scattering is used to probe the cluster morphology, and how it is modified by
reactant adsorption, heating, etc. Chemical properties are probed by a variety of
mass spectrometric methods. The goals are to understand the relationships between
cluster size, support interactions, electronic properties, and catalytic activity.
The goal of these experiments is to probe the correlations between supported cluster
size, morphology, electronic structure, adsorbate binding sites, support properties,
and catalytic activity, and thereby, to learn about catalyst active sites, mechanisms,
and approaches to improving activity/selectivity. We use size- and energy-selected
metal cluster ion deposition on well-characterized oxide surfaces to prepare model
catalysts, which are then studied using a variety of in situ techniques. The electronic
structure of the samples is probed by a combination of XPS, UPS, and INS. Low energy
ion scattering is used to probe the cluster morphology, and how it is modified by
reactant adsorption, heating, etc. Chemical properties are probed by a variety of
mass spectrometric methods. The goals are to understand the relationships between
cluster size, support interactions, electronic properties, and catalytic activity.
Effects of Site Size on Electrocatalytic Reactions
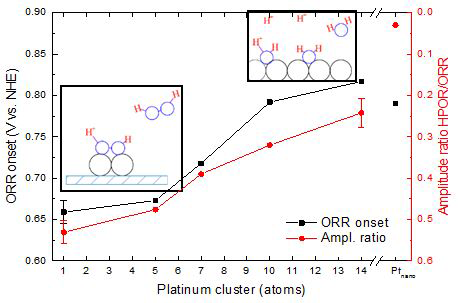 We have recently begun a project using cluster deposition to probe the effects of
catalyst site size on aqueous electrochemical reactions. Size-selected electrodes
are prepared by cluster deposition in UHV, characterized using surface analysis tools,
and then transferred without air exposure, to an antechamber where their activity
for electrochemical reactions such as oxygen reduction and ethanol oxidation can be
studied. Very strong effects of cluster size on both activity (current densities)
and product branching are observed. The figure summarizes recent results for the
oxygen reduction reaction (ORR) over Ptn/ITO electrodes. Note that the ORR onset potential is only modestly dependent on size,
however, the branching ratio between production of H2O and H2O2 (represented by the HPOR/ORR ratio) is strongly size dependent. We attribute this
to inability of the small clusters to dissociate O2, thus promoting H2O2 formation.
We have recently begun a project using cluster deposition to probe the effects of
catalyst site size on aqueous electrochemical reactions. Size-selected electrodes
are prepared by cluster deposition in UHV, characterized using surface analysis tools,
and then transferred without air exposure, to an antechamber where their activity
for electrochemical reactions such as oxygen reduction and ethanol oxidation can be
studied. Very strong effects of cluster size on both activity (current densities)
and product branching are observed. The figure summarizes recent results for the
oxygen reduction reaction (ORR) over Ptn/ITO electrodes. Note that the ORR onset potential is only modestly dependent on size,
however, the branching ratio between production of H2O and H2O2 (represented by the HPOR/ORR ratio) is strongly size dependent. We attribute this
to inability of the small clusters to dissociate O2, thus promoting H2O2 formation.
 Surface Chemistry and Emission Spectroscopy of Single Trapped Nanoparticles
Surface Chemistry and Emission Spectroscopy of Single Trapped Nanoparticles
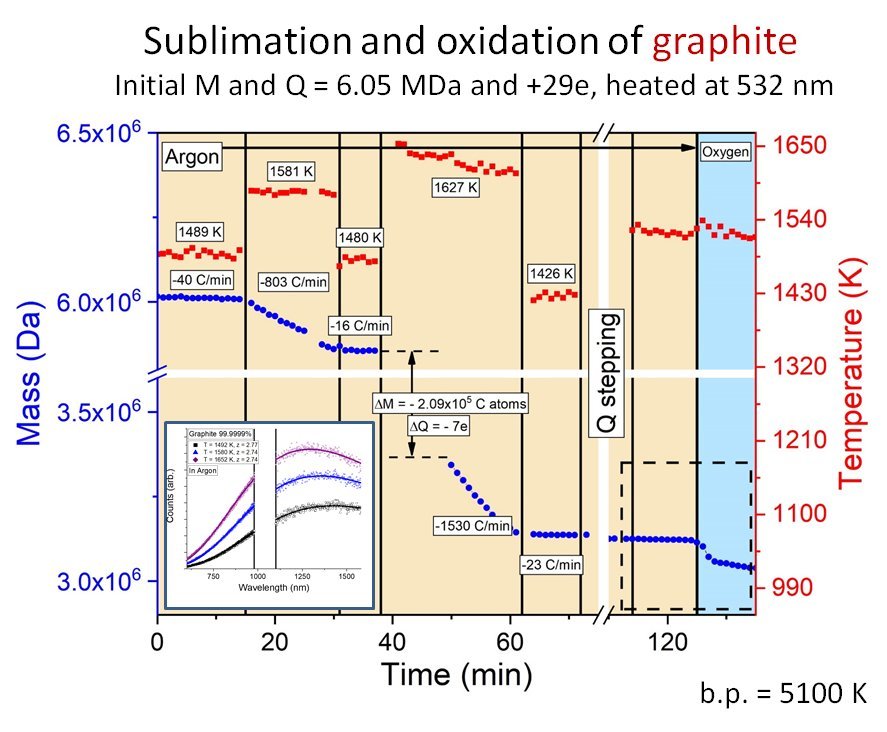
There are many problems in nanoscience where measurements average over a distribution of particle sizes or composition. Even if the particles are all the same size, there will be distributions of shape that affect the available surface sites, and therefore the surface chemistry and optical properties. Single particle measurement can be used to sort out the particle-to-particle variations in surface chemistry and emission spectra. In addition, it is even possible to use titration to count the number of reactive sites on individual nanoparticles. To do this, we have developed a method to probe single nanoparticles trapped in controlled atmospheres in a 3-d quadrupole trap. Particles of interest include carbon nanoparticles, metal nanoparticles, ultra-high temperature ceramic materials, semiconductor quantum dots, and even bioparticles such as viruses. The method allows us to determine the particle mass to precision approaching 1 ppm, allowing us to measure kinetics for mass-changing reactions (e.g. sublimation, oxidation) with near single atom precision. Particles can be laser heated to high or ultra-high temperatures, with temperature measured via the thermal (blackbody-like) emission spectrum.
Nanoparticle Fuel Additives
 We are exploring methods to produce high energy density nanoparticles with controlled
surface chemistry, for use as additives to fuels ranging from hydrocarbon jet fuels
to rocket propellants. The goals are to enhance combustion rates (catalytic surface
modifications) and to add energy density (boron or aluminum cores) while maintaining
high solubility in the fuel, and passivating the particles against premature air-oxidation
during storage and handling. Particles are generated by high energy milling, and characterized
by a combination of XPS, SEM, STEM, EDX, EELS, DLS, and combustion studies. The methods
may also be relevant to preparation of bio-compatible boron nanoparticles for use
in boron neutron capture therapy.
We are exploring methods to produce high energy density nanoparticles with controlled
surface chemistry, for use as additives to fuels ranging from hydrocarbon jet fuels
to rocket propellants. The goals are to enhance combustion rates (catalytic surface
modifications) and to add energy density (boron or aluminum cores) while maintaining
high solubility in the fuel, and passivating the particles against premature air-oxidation
during storage and handling. Particles are generated by high energy milling, and characterized
by a combination of XPS, SEM, STEM, EDX, EELS, DLS, and combustion studies. The methods
may also be relevant to preparation of bio-compatible boron nanoparticles for use
in boron neutron capture therapy.
Links to (other) Useful Stuff