Scott L. Anderson
 PHYSICAL, ANALYTICAL & MATERIALS CHEMISTRY
PHYSICAL, ANALYTICAL & MATERIALS CHEMISTRY
Distinguished Professor
Henry Eyring Presidential Endowed Chair
B.A. Rice University, 1977
Ph.D. University of California at Berkeley, 1981
Postdoctoral, Stanford University, 1981-83
ORCID 0000-0001-9985-8178
Phone: (801) 585-7289
Office: 1224 HEB
Email: anderson@chem.utah.edu
Research Group
Publications
Activities & Awards
- Chair, Div. Chemical Physics, American Physical Chemistry (2018-2019)
- ACS Physical Division Award in Experimental Physical Chemistry (2016)
- Robert W. Parry Teaching Award (2015)
- Associate Director for Surface Analysis and Nano-imaging, Utah Nanofab (2014 - )
- Fellow of the American Association for the Advancement of Science (2011)
- Distinguished Scholarly and Creative Research Award (2007)
- Fellow of the American Physical Society (2005)
- Visiting Scientist, Inst für Physik, Univ. Chemnitz (2004)
- Japan Society for the Promotion of Science Senior Invitation Fellowship (2002-2003)
- Professeur Invité, Université de Paris-Sud (1990-91)
- Visiting Scientist, Fakultät für Physik, Freiburg (1990)
- Camille and Henry Dreyfus Foundation Teacher-Scholar
- Japan Society for the Promotion of Science Fellow (1989-1990)
- Alfred P. Sloan Foundation Research Fellow (1988)
- Member of the Nano Institute of Utah
|
|
![Hypergolic ignition of boron nanoparticles in [MAT][DCA]](/_resources/images/faculty/anderson/hypergolic.png) |
Research Interests
The theme of my research is nanoparticle surface chemistry, with activities in four main areas:
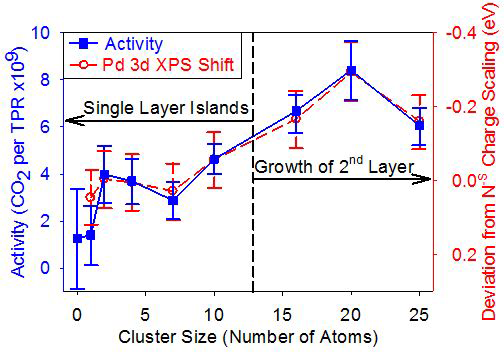
- Size-selected cluster deposition and size-effects on catalysis: Effects of electronic and geometric structure and cluster-support binding on activity.
- Effects of site size on electrocatalysis: Inherent effect of catalytic site size, aqueous electrochemistry without air exposure, high mass activity model electrodes.
- Single nanoparticle trapping mass spectrometry to study surface chemistry and optical properties with ppm size resolution: Size effects, new approaches to ultra-high temperature surface chemistry, light interactions with individual particles.
- Use of surface chemistry to control size and reactivity properties of high energy density nanoparticles for fuel/propellant applications: Reactant-assisted size reduction, air-stability via capping chemistry, effects of particle size, surface chemistry, and loading on ignition behavior.
See our Research Group web page for more information.