Nanoparticle Fuel Additives
Issues relating to nanoparticulate fuel additives
 The goal is to design and produce nanoparticle additives for jet/rocket fuels and
pyrotechnic solids, that significantly increase the energy density, while maintaining
or improving combustion rates. Applications range from hypersonic flight to long duration/micro
satellite propulsion. There are a number of tough issues that all must be addressed
to make this happen. We are aiming for a simple and inexpensive synthesis method for
nanoparticles that will simultaneously address the following issues:
The goal is to design and produce nanoparticle additives for jet/rocket fuels and
pyrotechnic solids, that significantly increase the energy density, while maintaining
or improving combustion rates. Applications range from hypersonic flight to long duration/micro
satellite propulsion. There are a number of tough issues that all must be addressed
to make this happen. We are aiming for a simple and inexpensive synthesis method for
nanoparticles that will simultaneously address the following issues:
1. High Energy Density
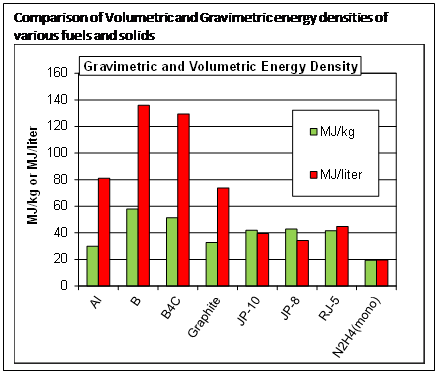
The goal is to load liquid or solid fuels with solid particles that add significantly to the energy density. The figure compares the energy density of typical hydrocarbon fuels and hydrazine (rocket fuel), with some potential solid fuel additives. Note that aluminum has significantly increased energy/volume, but lower energy/mass compared to hydrocarbons. Boron and boron-rich solids are significantly better than hydrocarbons on both volume and mass bases. We are working on both aluminum and boron nanoparticles, because each has advantages for different applications. In the following, I will use the term metal to refer to both aluminum and boron, a semi-metal.2. Heterogeneous kinetics
Hydrocarbons burn by vaporizing, mixing with oxidizer, and then burning in the gas phase. Boron is refractory, and even aluminum has a vaporization temperature >2000K. Therefore, reaction rates will tend to be limited by diffusion to and from the metal particle surfaces. To make this rate as fast as possible, it is important to disperse the metal as nanoparticles, and to somehow keep the particles dispersed as the fuel/nanoparticle mixture burns3. Native oxide passivation
One major problem with combustion of both boron and aluminum (most metals, in fact) is that the metal surfaces are passivated by a native oxide layer a few nanometers thick. This oxide greatly retards ignition of the metal. In addition, if the particle size is reduced into the nanoscale, the oxide (which is just dead weight) becomes a larger and larger fraction of the total mass, with corresponding reduction in energy density. Particles can easily be generated without an oxide coating, however, if they are not protected somehow, they ignite (sometimes explosively) upon contact with air.4. Air-stable, un-oxidized nanoparticles
For obvious reasons, most applications require that fuel be stable in contact with air, during storage, etc. Therefore, one of our goals is to generate particles that are un-oxidized, yet air stable.5. High solubility/dispersibility in fuels, solid propellants.
For liquid fuels, we want our additives to be highly soluble (i.e., forming stable, high density suspensions) in the fuel. For solid propellants or pyrotechnics, the issue is mixability the ability to load large densities of nanoparticles into the binder. In both cases, the problem requires that the surface free energy of the particles in contact with the fuel or binder be as low as possible. This is done by controlling the nanoparticle surface chemistry.6. Ignition catalysts
Another idea we have demonstrated, is the ability to coat the surface of the nanoparticles with patches of a combustion catalyst or ignition aid. For example, we have generated fuel-soluble boron nanoparticles that have a partial coating of CeO2.7. Cost and scalability
There are many elegant gas-phase and solution-phase nanoparticle synthesis methods, giving great control over particle size, surface chemistry, etc. The problem is that they tend to use expensive reagents, and/or complex processing. We have focused on single step, single pot synthesis methods based on ball milling, which is a cheap and readily scalable method. The trick is to pick the right conditions, including capping agents, to generate particles in the desired size range with the desired surface chemistry. The starting materials are simply the bulk metal, plus capping agents and dispersing agents, all of which are inexpensive. Even laboratory scale mills can produce many grams/day.
8. Combustion and other properties
 You have to test these things, and we collaborate with a number of groups at the Naval
Research Lab, University of Hawaii, University of Alabama, and Purdue University,
and have provided particle-loaded fuels for tests ranging up to operation in a 14
HP turbine engine. In addition, we have developed a turbulent flame calorimeter capable
of accurately measuring total power output from flames such as the boron-loaded flame
shown in the figure (flame is about 8 long). We also make extensive use of analytical
tools such as scanning transmission electron microscopy (STEM), scanning electron
microscopy (SEM), dynamic light scattering (DLS), X‑ray photoelectron spectroscopy
(XPS), NMR, and FT-IR spectroscopy to understand the nature of the particle surfaces.
You have to test these things, and we collaborate with a number of groups at the Naval
Research Lab, University of Hawaii, University of Alabama, and Purdue University,
and have provided particle-loaded fuels for tests ranging up to operation in a 14
HP turbine engine. In addition, we have developed a turbulent flame calorimeter capable
of accurately measuring total power output from flames such as the boron-loaded flame
shown in the figure (flame is about 8 long). We also make extensive use of analytical
tools such as scanning transmission electron microscopy (STEM), scanning electron
microscopy (SEM), dynamic light scattering (DLS), X‑ray photoelectron spectroscopy
(XPS), NMR, and FT-IR spectroscopy to understand the nature of the particle surfaces.
Recent Results
Un-oxidized, air-stable, catalyst-coated boron nanoparticles
Un-oxidized fuel/propellant soluble Al nanoparticles
Details on boron:
1. Van Devener, B., Perez, J. P. L., Anderson, S. L., Air-stable, Unoxidized, Fuel-Soluble
Boron Nanoparticles
Journal of Materials Research, 2009. 24 p. 3462-3464.
2. Van Devener, B., et al., Oxide-Free, Catalyst-Coated, Fuel-Soluble, Air-Stable
Boron Nanopowder as Combined Combustion Catalyst and High Energy Density Fuel. Energy
and Fuels, 2009. 23: p. 6111-6120
