Shedding Light on Tetracyanoethylene Dimerization
Joel Miller Publishes in Chemistry: A European Journal
Professor Joel Miller recently published his paper “Cation Dependence of the Dimerization
Enthalpy for A2[tetracyanoethylene]2 (A=NMe4, Mepy, NEt4) Possessing a Long, Multicenter Bond” in Chemistry: A European Journal. It was written in collaboration with Adora G. Graham and Matvey V. Fedin and “Dedicated
to Edel Wasserman on the occasion of his 85th birthday, and to the memory of the DuPont
Central Research Department—the birthplace of cyanocarbon chemistry (1957–2016).”
Abstract: [TCNE].− (TCNE=tetracyanoethylene) has been isolated as D2h π–[TCNE]22− possessing a long, 2.9 Å multicenter 2-electron-4-center (2e−/4c) C−C bond, and as C2 π–[TCNE]22− possessing a longer, 3.04 Å multicenter 2e−/6c (4 C+2 N atoms) bond. Temperature-dependent UV/Vis spectroscopic measurements
in 2-methyltetrahydrofuran (MeTHF) has led to the determination of the dimerization,
2[TCNE].−⇌π–[TCNE]22−, equilibrium constants, Keq(T), [[TCNE]22−]/[[TCNE].−]2, enthalpy, ΔH, and entropy, ΔS, of dimerization for [Mepy]2[TCNE]2 (Mepy=N-methylpyridinium, H3CNC5H5+) possessing D2h π–[TCNE]22− and [NMe4]2[TCNE]2 possessing C2 π–[TCNE]22−conformations in the solid state; however, both form D2h π–[TCNE]22− in MeTHF solution. Based on ΔH=−3.6±0.1 kcal mol−1 (−15.2 kJ mol−1), and ΔS=−11±1 eu (−47 J mol−1 K−1) and ΔH=−2.4±0.2 kcal mol−1 (−10.2 kJ mol−1), and ΔS=−8±1 eu (−32 J mol−1 K−1) in MeTHF for [NMe4]2[TCNE]2 and [Mepy]2[TCNE]2, respectively, the calculated Keq(298 K) are 1.6 and 1.3 m−1, respectively. The observed Keq (145 K) are 3 and 2 orders of magnitude greater for [NMe4]2[TCNE]2 and [Mepy]2[TCNE]2, respectively. The Keq(130 K) is 4470, 257, ≈0.8, and ≪0.1 m−1 for [NMe4]2[TCNE]2, [Mepy]2[TCNE]2, [NEt4]2[TCNE]2, and [N(nBu)4]2[TCNE]2, respectively, decreasing with increasing cation size. At standard conditions and
below ambient temperature the equilibrium favors the dimer for the NMe4+ and Mepy+ cations. From the decreasing enthalpy, NMe4+>Mepy+, along with the decrease in dimer formation Keq(T) as NMe4+>Mepy+>NEt4+>N(nBu)4+, the dimer bond energy decreases with increasing cation size in MeTHF. This is attributed
to a decrease in the [A]+⋅⋅⋅[TCNE]− attractive interactions with increasing cation size. Solid state UV/Vis spectroscopic
determinations of [NMe4]2[TCNE]2 are reported and compared to D2h π–[TCNE]22− conformers. The feasibility and limitations of temperature-dependent electron paramagnetic
resonance (EPR) measurements for the determination of Keq(T) are also discussed.
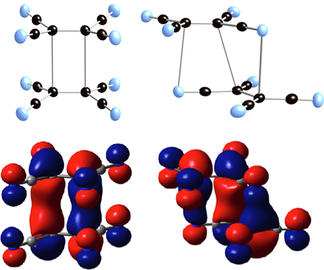 Structure (50 % electron density ORTEP) showing the intradimer bonding components
and HOMO for D2h π–[TCNE]22− possessing a 2.9 Å, 2e−/4c C−C bond (a),
Structure (50 % electron density ORTEP) showing the intradimer bonding components
and HOMO for D2h π–[TCNE]22− possessing a 2.9 Å, 2e−/4c C−C bond (a),
and C2 π–[TCNE]22− possessing a 3.04 Å 2e−/6c C−C (4 C+2 N atoms) bond (b) (adapted from Ref. [9]).
