Ongoing and Future Work in the Rainier Group
Research in the Rainier group emphasizes studies in the field of chemical synthesis and the exploration of the properties of synthetic targets. Within this discipline, we are particularly fascinated by the interplay between structure (biological activity), total synthesis, and reaction development.
Current Projects
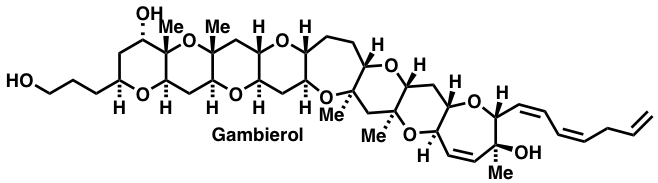
Antibiotics/Virulence Inhibitors:
Discorhabdin Z and taxodone and related analogs serve as targets that affect bacterial growth. Our path to these involve unique amidoindoloquinone and bis-aryl cycloalkenone photoelectrocyclization
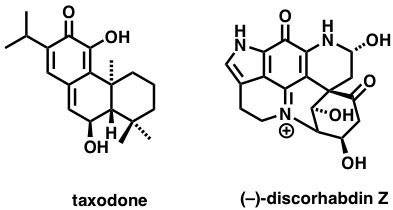
Macular Degeneration:
Very Long Chain Polyunsaturated Fatty Acids (VLC-PUFA’s) and related analogs are potential therapeutics eye diseases. Our synthetic approach to these agents includes either fatty acid coupling reactions or alkyne coupling and reduction sequences.
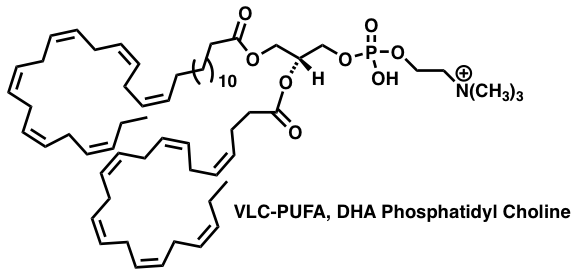
Ion Channel Effectors:
Fused Polycyclic Ethers like gambierol and related analogs have a unique mode of binding to ion channels. Central to our approach to these agents are two-directional Olefinic-Ester Cyclization (OLEC) Reactions.