Wendy Hibbs

B.S. Eastern Kentucky University
Ph. D University of Utah (2002)
1-D Metal-Radical Chains: Porphyrin Systems
 The study of magnetically ordered organic materials is a growing area of contemporary
chemistry, and establishment of structure-function relationships is critical to its
advancement. [Fe(C5Me5)2]�+[TCNE]�- (TCNE = tetracyanoethylene) was the first organic-containing
molecule-based magnet characterized, and has an ordering temperature, Tc, of 4.8 K.
Improvements led to the discovery of V(TCNE)x�y(solvent), the first room temperature
organic magnet (Tc ~ 400 K), and later the magnetically ordered Mn, Fe, Ni, and Co
analogs. More recently, studies have focused on a class of metallomacrocycles and
[TCNE]�--based magnets exemplified by [MnTPP][TCNE]�2PhMe, (TPP = meso-tetraphenylporphinato).
The [MnTPP][TCNE] family provides a broad foundation to test known 1-D magnetic models
and develop new theories. Metalloporphyrin systems are appealing in that they are
easily modified enabling thorough testing of structure-function relationships with
the ultimate goal of obtaining predictable and tunable physical properties.
The study of magnetically ordered organic materials is a growing area of contemporary
chemistry, and establishment of structure-function relationships is critical to its
advancement. [Fe(C5Me5)2]�+[TCNE]�- (TCNE = tetracyanoethylene) was the first organic-containing
molecule-based magnet characterized, and has an ordering temperature, Tc, of 4.8 K.
Improvements led to the discovery of V(TCNE)x�y(solvent), the first room temperature
organic magnet (Tc ~ 400 K), and later the magnetically ordered Mn, Fe, Ni, and Co
analogs. More recently, studies have focused on a class of metallomacrocycles and
[TCNE]�--based magnets exemplified by [MnTPP][TCNE]�2PhMe, (TPP = meso-tetraphenylporphinato).
The [MnTPP][TCNE] family provides a broad foundation to test known 1-D magnetic models
and develop new theories. Metalloporphyrin systems are appealing in that they are
easily modified enabling thorough testing of structure-function relationships with
the ultimate goal of obtaining predictable and tunable physical properties.
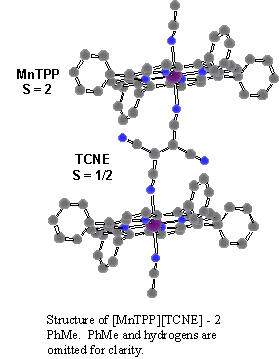
The electron-transfer salt [MnIIITPP][TCNE]�2PhMe, (see figure) was first reported by Basolo, et al.¹ with a room temperature moment of 4.88 meff and a moment approaching 6.6 meff at 77 K. Upon further examination by Miller, et al., this material was observed to undergo 3-D magnetic ordering below 14 K.² Structural characterization of this and other substituted systems show trans-m2-N-m-bonding of [TCNE]�- to the Mn³+ center of the porphyrin forming 1-D coordination polymers consisting of parallel chains of alternating spin sites of spin = 2 and spin = ½. Both the intra- and inter-chain coupling of these spin sites are responsible for the 3-D magnetic ordering. The strength of the coupling is influenced by the physical distance between spin sites and thereby affected by structural modifications, which may be implemented by intercalation of various solvents or by simple substitution.
References
(1) (a) Summerville, D.A.; Cape, T.W.; Johnson, E.D.; Basolo, F. Inorg. Chem. 1978, 17, 3297. (b) Jones, R.D.; Summerville, D.A.; Basolo, F. Acta Chem. Scan.1978, A32, 771.
(2) (a) Miller, J.S.; Calabrese, J.C.; McLean, R.S.; Epstein, A.J. Adv. Mater. 1992, 4, 498. (b) Zhou, P.; Morin, B.G.; Epstein, A.J.; McLean, R.S.; Miller, J.S. J. Appl. Phys. 1993, 73, 6569.
