Chemistry of High Energy Density Fuels
Motivations:
A. Develop methodology, including low energy guided beam scattering methods, as a structural probe for identifying metastable product isomers. Some of the methods may also be applicable to other isomer detection problems such as chiral recognition.
B. Probe pyrolysis (thermal decomposition) chemistry and initial stages of oxidation chemistry for high energy density fuels molecules, including actual fuels such as JP-10 and highly strained potential fuels such as cubane and quadricyclane. Develop methodology for tracking sequential decomposition of complex hydrocarbon fuels. Develop rapid evaluation methods for synthetic strained high energy fuels.
Techniques: Micro Flow tube reactor kinetics, gentle ionization methods, low energy, high energy resolution scattering techniques for isomer "titration".
Funding - ONR (Mechanics and energy conversion) ONR Home Page
Typical Results: (Note: the slide shows may be too big if you have a slow internet connection).
JP-10 pyrolysis - a slide show
Decomposition of Strained C7H8 isomers.
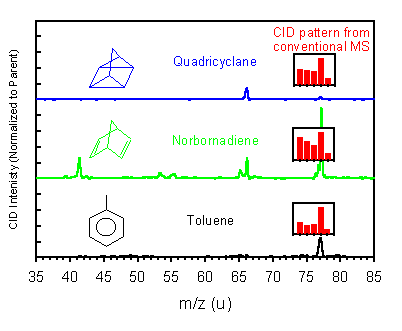
Quadricyclane, Norbornadiene, and Toluene are three C7H8 isomers (see figure), quadricyclane being quite strained due to the cyclopropane rings. Quadricyclane and other strained molecules are being examined for use in next-generation aircraft engines because they have substantially higher volume energy densities than normal hydrocarbons. Not much is known about how these molecules decompose at high temperatures, and we have developed a method to study this. One of the strategies is to funtionalize the molecules to control chemical/physical properties, and a key feature of our method is the ability to study the very small samples that are initially available from the synthetic chemists.
Mass spectrometry has a hard time differentiating strained molecules from other isomers because they tend to rearrange easily. The red bar-graph inserts show the collision-induced dissociation pattern found in a conventional instrument. We have found that low energy scattering of the molecular ions from these isomers is able to clearly distinguish them. The fragmentation pattern also gives some insight into the decomposition mechanism of the different isomers.
By using the mass spectrometry tricks developed, we can then follow the chemistry of the different isomers. The figure below shows CI mass spectra of the Quadricyclane isomer. The sample has been diluted in argon and passed through a small flow tube reactor at increasing temperatures. From 600-700K, the quadricylane appears to decompose to first to norbornadiene, then on to toluene and C5H6 + acetylene. At higher temperatures, this decomposition mechanism is replaced by a polymerization reaction. Curiously, if helium is used as the carrier gas, rather than argon, no polymerization is observed. These results are important in understanding storage, injection, and combustion of quadricyclane in high performance engines.

FIGURE Caption Breakdown of neutral quadricyclane (QC) as a function temperature in a micro-flowtube reactor. Up to about 500K QC is stable for the 5 millisecond reactor residence time. By 673K, QC is starting to decompose. From comparisons to similar data for norbornadiene and other C7H8 isomers, we can see that QC is isomerizing to norbornadiene, which further decomposes by both isomerization to toluene and by C2H2 loss (retro-Diels-Alder reaction). At higher temperatures the QC decomposes almost completely by oligamerization, followed by loss of small hydrocarbon fragments.
