Combustion Chemistry
 We have a number of activities in the combustion/fuels/propulsion area, some of which
are summarized in other links off the main page Fundamental pyrolysis and oxidation chemistry of JP-10. Petroleum fuels are complex mixtures, making it very difficult to carry out experimental
and theoretical studies of their fundamental combustion chemistry. JP-10 (C10H16)
is a pure, single component synthetic jet fuel used because it combines high energy
density with a broad range of useable temperatures. Like most hydrocarbon molecules
of this size, its pyrolysis and oxidation chemistry are not understood in any detail,
but unlike petroleum fuels (diesel, other JP fuels), it is feasible to try to develop
a comprehensive combustion model. My group has reported a number of studies of JP-10
pyrolysis and oxidation, with and without catalysts, and currently is working with
groups at Reaction Engineering Inc (Salt Lake City), and NJIT (Prof. Joe Bozzelli)
to develop a JP-10 combustion model. The U of Utah contribution to this effort has
two components.
We have a number of activities in the combustion/fuels/propulsion area, some of which
are summarized in other links off the main page Fundamental pyrolysis and oxidation chemistry of JP-10. Petroleum fuels are complex mixtures, making it very difficult to carry out experimental
and theoretical studies of their fundamental combustion chemistry. JP-10 (C10H16)
is a pure, single component synthetic jet fuel used because it combines high energy
density with a broad range of useable temperatures. Like most hydrocarbon molecules
of this size, its pyrolysis and oxidation chemistry are not understood in any detail,
but unlike petroleum fuels (diesel, other JP fuels), it is feasible to try to develop
a comprehensive combustion model. My group has reported a number of studies of JP-10
pyrolysis and oxidation, with and without catalysts, and currently is working with
groups at Reaction Engineering Inc (Salt Lake City), and NJIT (Prof. Joe Bozzelli)
to develop a JP-10 combustion model. The U of Utah contribution to this effort has
two components.
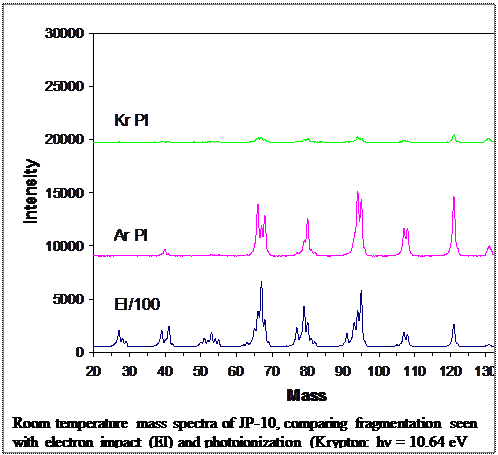
- Ab initio molecular dynamics of unimolecular and bimolecular reactions: For a complex molecule like JP-10 it can be difficult to anticipate all possible reaction pathways that might become significant under different reaction conditions. As an aid to mechanism development, we are running extensive sets of ab initio trajectories, with forces calculated at the B3LYP/6-31G* level of theory. Each trajectory takes ~500 - 600 cpu hours, and hundreds are required for each set of reaction conditions. Trajectories have been run for JP-10 thermal unimolecular decomposition at various temperatures, for unimolecular decomposition of each C10H15 radical, and bimolecular reactions are Text Box: Room temperature mass spectra of JP-10, comparing fragmentation seen with electron impact (EI) and photoionization (Krypton: hν = 10.64 eV and 10.03 eV, Argon: hν = 11.83 eV and 11.62 eV)running being studies between JP-10 and OH and other combustion radicals. Numerous reaction pathways, have been identified. Some are relatively obvious, and would have been explored based on chemical intuition. Others are considerably more complex, and would have been omitted from the model. Such calculations are only possible due to generous allocations of time on DOD supercomputers.
- Flow tube studies of JP-10 breakdown under various conditions: We use a small flow tube reactor, coupled to a mass spectrometer for product detection, to study pyrolysis, oxidation, and catalytic combustion of JP-10 and other fuel molecules on a few millisecond time scale. We recently added a home-made resonance lamp to allow photoionization (PI) to be used in detection. This is rather important for JP-10, as it tends to fragment extensively using conventional ionization methods such as electron impact or proton transfer chemical ionization. PI at various wavelengths is necessary to ensure detection of both low ionization energy weakly-bound species like JP-10, and well as high ionization energy products such as water or CO.
Other activities in the combustion area include both fundamental and more realistic studies of catalytic processes relating to ignition and propagation of combustion reactions, and development of nanoparticulate additives for fuels ranging from hydrocarbons to rocket propellants. These activities are described in other sections of this web site.
Green, R. J., S. Nakra and S. L. Anderson (2005). Thermal decomposition of JP-10 studied by microflow tube pyrolysis mass spectrometry. Combustion Processes in Propulsion. G. D. Roy. London, Butterworth Heinemann: 480.
Nakra, S., R. J. Green and S. L. Anderson (2002). Chemistry of JP-10 Relating to PDE Combustion and Diagnostics. 15th ONR Propulsion Meeting, Washington, DC.
Nakra, S., R. J. Green and S. L. Anderson (2006). "Thermal decomposition of JP-10 studied by micro-flowtube pyrolysis-mass spectrometry." Combust. Flame 144: 662-674.
Van Devener, B. and S. L. Anderson (2006). "Breakdown and Combustion of JP-10 Fuel Catalyzed by Nanoparticulate CeO2 and Fe2O3." Energy and Fuels 20: 1886-1894.
