Publications
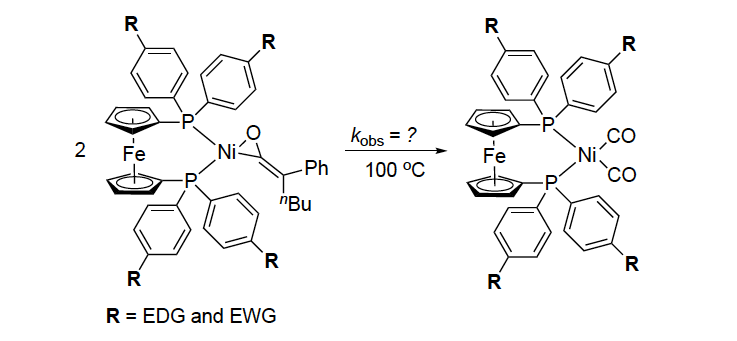
N. Al, R. M. Stolley, N. D. Staudaher, R. T. Vanderlinden, J. Louie “Electronic Effect of Ligands on the Stability of Nickel-Ketene Complexes” Organometallics 2018, accepted for publication
A. L. Clevenger, R. M. Stolley, N. D. Staudaher, N. Al, A. Rheingold, R. T. Vanderlinden, J. Louie “A Comprehensive Study of the Reactions Between Chelating Phosphines and Ni(COD)2”Organometallics 2018, ASAP, doi:10.1021/acs.organomet.8b00438

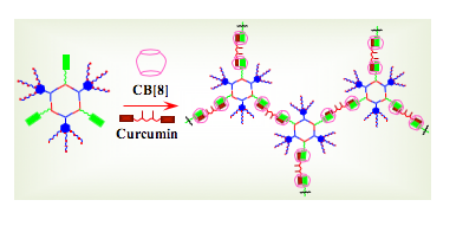
S. Datta, S. K. Misra, M. L. Saha, N. Lahiri, J. Louie, D. Pan, P. J. Stang “Orthogonal Self-Assembly of an Organoplatinum(II) Metallacycle and Cucurbit[8]uril That Delivers Curcumin to Cancer Cells”Pro. Nat. Acad. Sci.2018, 115, 8087-8092
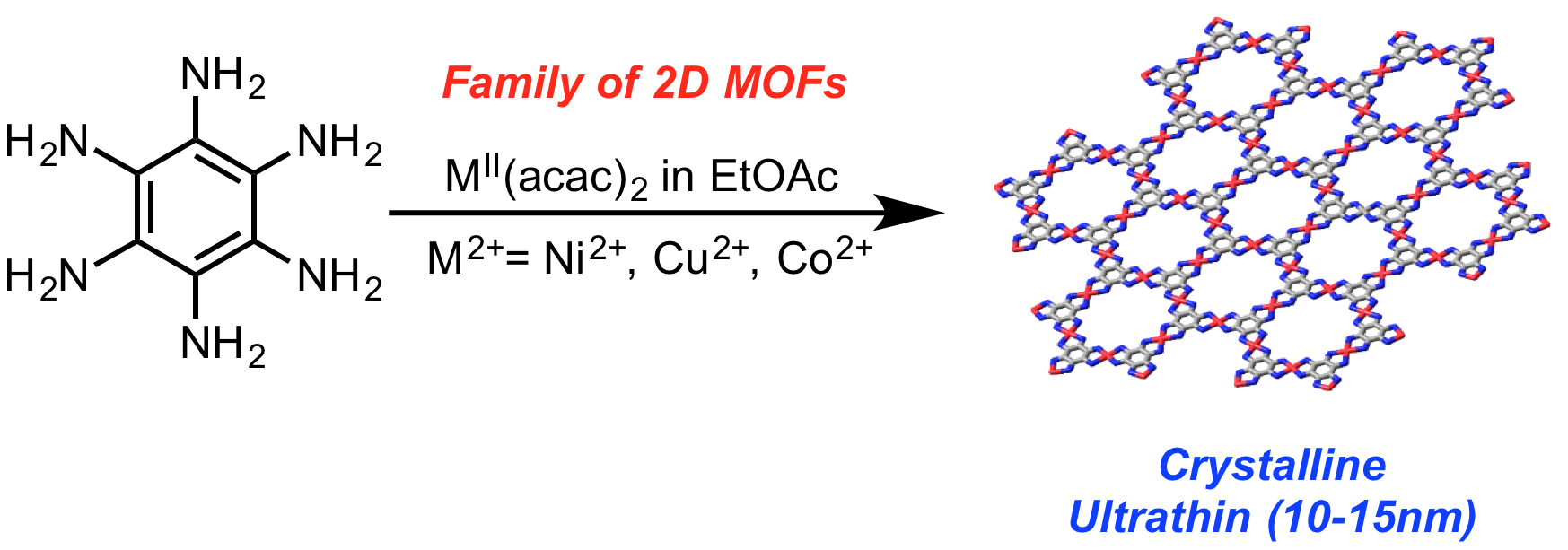
N. Lahiri, R. Tsuchikawa, N. Lotfizadeh, V. V. Deshpande, J. Louie “Hexaminobenzene as a Building Block for Family of 2D Coordination Polymers” J. Am. Chem. Soc., 2017, 139 (1), pp 19–22
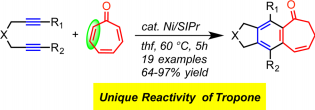
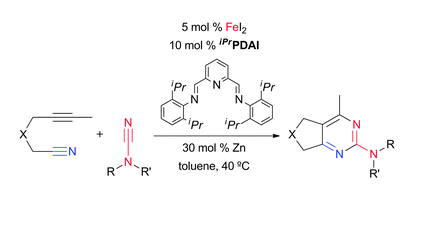
N. A. Spahn, M. H. Nguyen, J. Renner, J. Louie “Regioselective Iron-catalyzed [2+2+2] Cycloaddition Reaction forming 4, 6-Disubstituted 2-Aminopyridines from Terminal Alkynes and Cyanamides” J. Org. Chem. 2017, 82, 234-242

Nicholas D. Staudaher, Atta M. Arif, and Janis Louie. "Synergy between Experimental and Computational Chemistry Reveals the Mechanism of Decomposition of Nickel–Ketene Complexes" J. Am. Chem. Soc., 2016 138 (42), pp 14083–14091
Nicholas D. Staudaher, Joseph Lovelace, Michael P. Johnson, and Janis Louie "Preparation of Aryl Alkyl Ketenes" Org. Synth. 2017, 94, 1-15
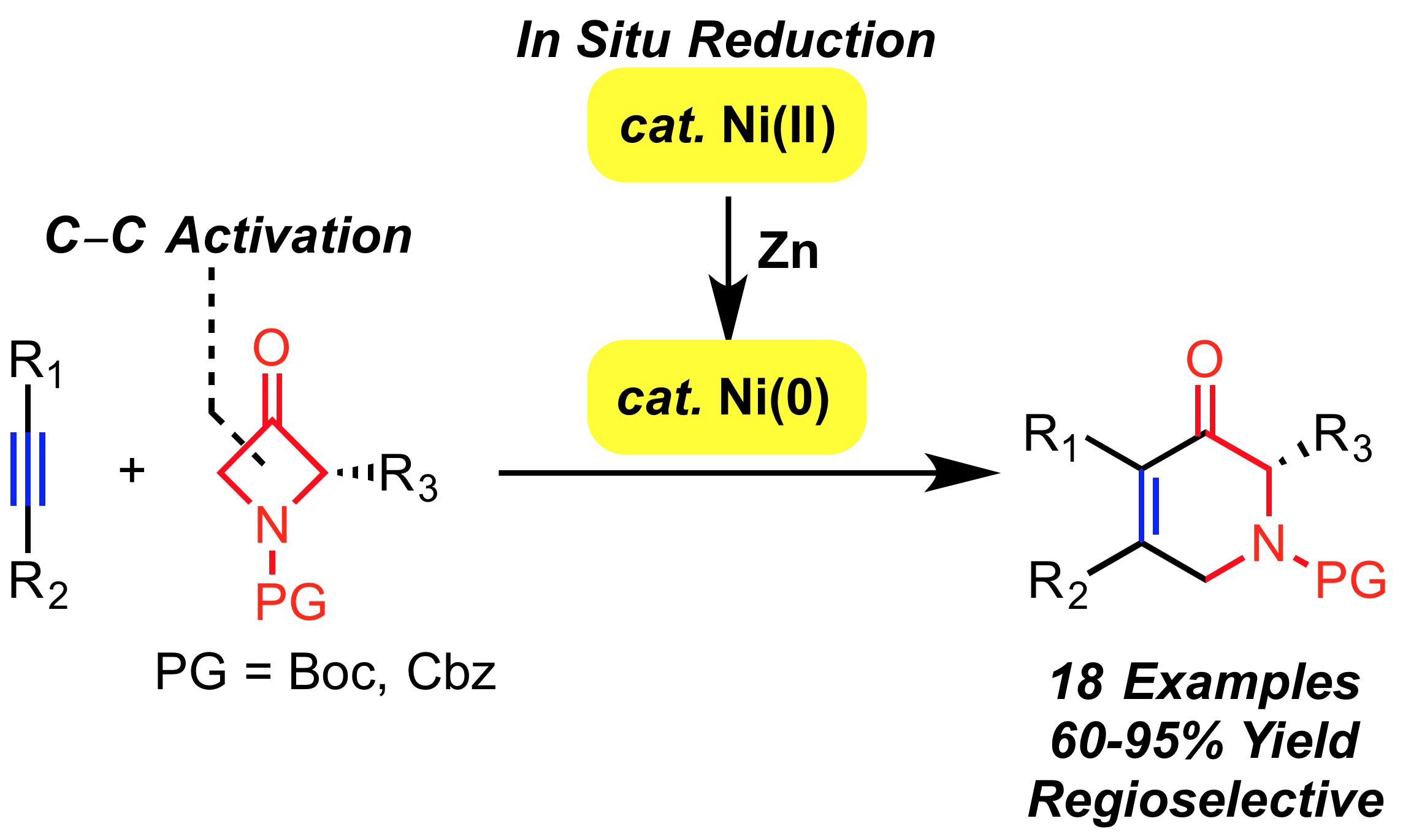
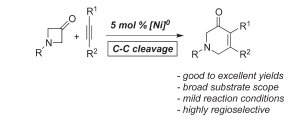
A. Thakur, J. Evangelista, J. Louie “An in situ Ni-Catalyzed Approach to Substituted Piperidones”J. Org. Chem. 2015, 80, 9951-9958.
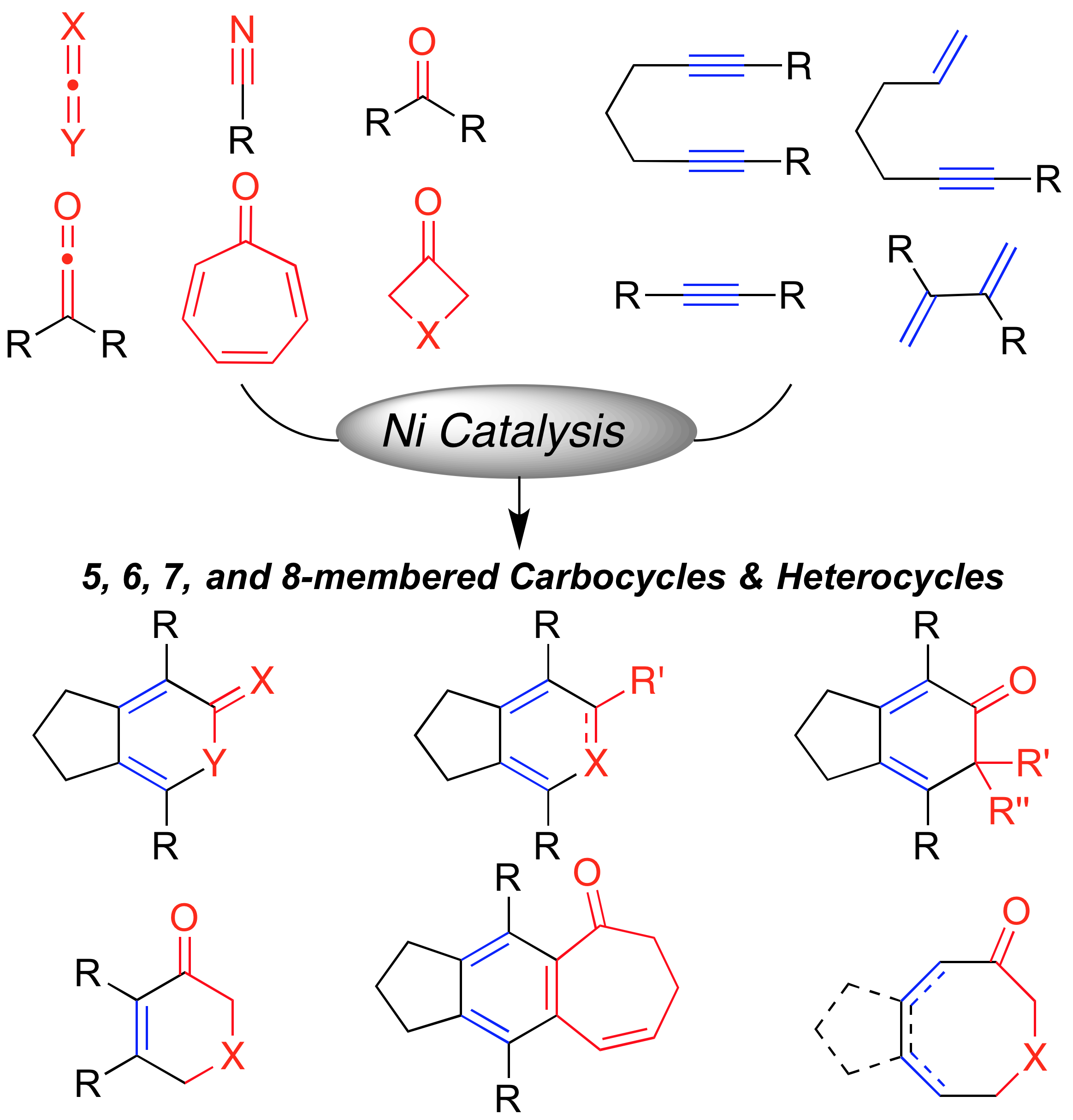
A. Thakur, J. Louie “Advances in Nickel-Catalyzed Cycloaddition Reactions To Construct Carbocycles and Heterocycles”Acc. Chem. Res. 2015, 48, 2534-2365; Invited Contribution to a Special Issue (Earth Abundant Metals in Homogeneous Catalysis).
Y. Zhong, N. A. Spahn, R. M. Stolley, M. H. Nguyen, J. Louie “3,5-Disubstituted-2-Aminopyridines via Ni-catalyzed Cycloaddition of Terminal Alkynes and Cyanamides”Synlett 2015, 26, 307-312; Invited Contribution to a Special Issue (Cluster Report on Catalysis with Sustainable Metals).
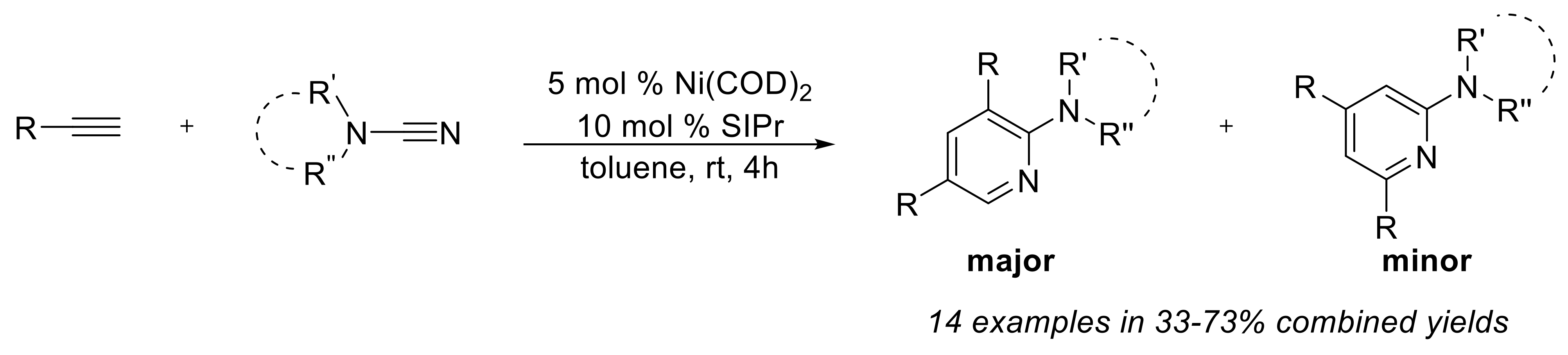
P. Kumar, A. Thakur, X. Hong, K. N. Houk, J. Louie “[Ni(NHC)]-catalyzed Cycloaddition of Diynes and Tropone: Apparent Enone Cycloaddition Involving an 8p Insertion”J. Am. Chem. Soc. 2014, 136, 17844-17851.
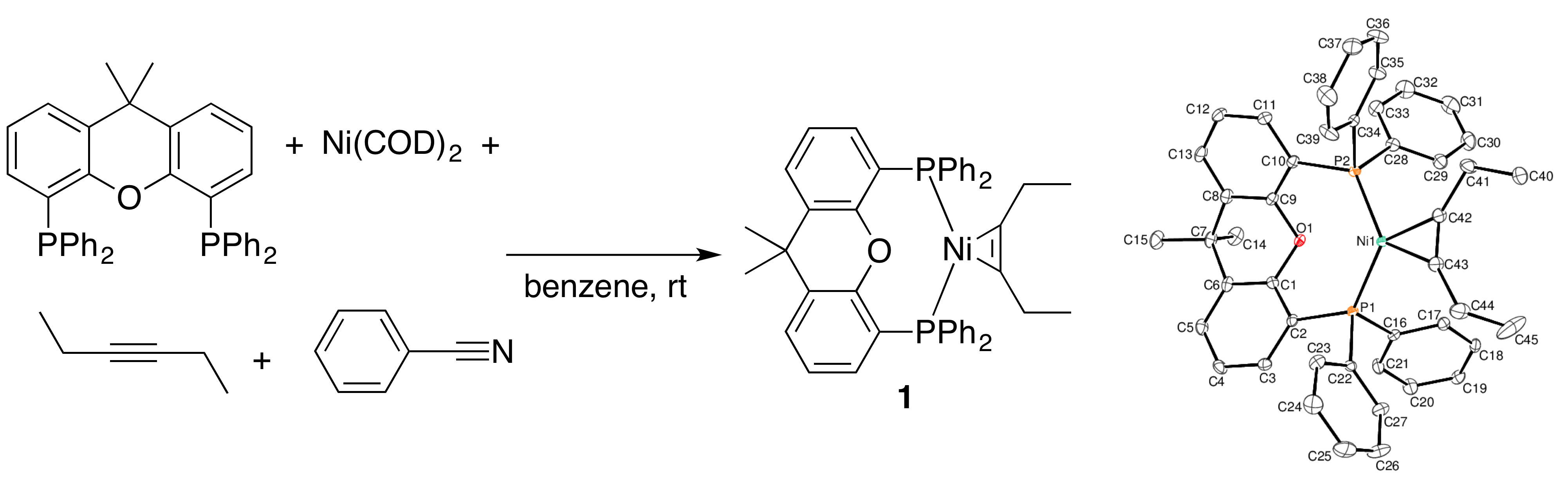
N. D. Staudaher, R. M. Stolley, J. Louie “Synthesis, Mechanism of Formation, and Catalytic Activity of Xantphos Nickel p-Complexes”Chem. Commun. 2014, 50, 15577-15580.
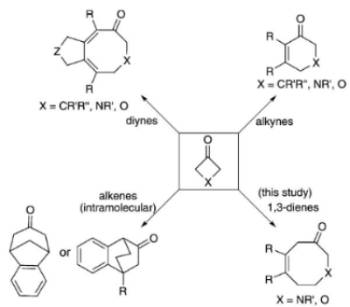
A. Thakur, M. E. Facer,† J. Louie “Nickel Catalyzed Cycloaddition of 1,3-Dienes with 3-Azetidinones and 3-Oxetanes” Angew. Chem. Int. Ed. 2013, 52, 12161-12165. PubMed PMID: 24573793; PubMed Central PMCID: PMC4113093.
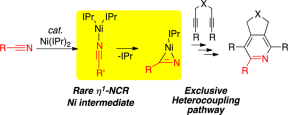
R. M. Stolley, H. A. Duong, J. Louie “Mechanistic Evaluation of the Ni(IPr)2-Catalyzed Cycloaddition of Alkynes and Nitriles to Afford Pyridines: Evidence for the Formation of a Key h1-Ni(IPr)2(RCN) Intermediate”Organometallics 2013, 32, 4952-4960. PubMed PMID: 25214702; PubMed Central PMCID: PMC4159214.
T. K. Lane, M. H. Nguyen, N. Spahn, J. Louie “The Iron-Catalysed Construction of 2-Aminopyrimidines from Alkynenitriles and Cyanamides”Chem. Commun. 2013, 49, 7735-7737. PubMed PMID: 23877441; PubMed Central PMCID: PMC4144345.
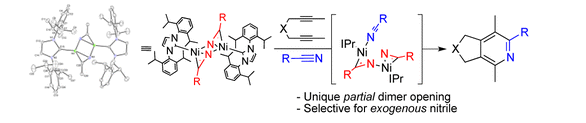
R. M. Stolley, H. A. Duong, D. R. Thomas, J. Louie “The Discovery of [Ni(IPr)RCN]2 Species and their Role as Cycloaddition Catalyts for the Formation of Pyridines”J. Am. Chem. Soc. 2012, 134, 15154-15162. PubMed PMID: 22917161; PubMed Central PMCID: PMC3480329.
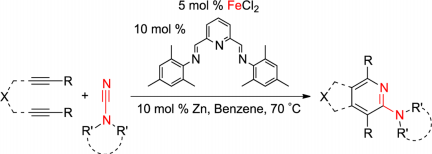
T. K. Lane, B. R. D’Souza, J. Louie “2-Aminopyridines from Iron-Catalyzed Cycloaddition of Diynes and Cyanamides”J. Org. Chem. 2012, 77, 7555-7563. PubMed PMID: 22845666; PubMed Central PMCID: PMC3480319.
P. Kumar, K. Zhang, J. Louie “An Expeditious Route to Eight-Membered Heterocycles by Nickel-Catalyzed Cycloaddition: Low-Temperature Csp2-Csp3 Bond Cleavage”Angew. Chem. Int. Ed. 2012, 51, 8602-8606. PubMed PMID: 22806996; PubMed Central PMCID: PMC3557805.
P. Kumar, J. Louie “A Single Step Approach to Highly Substituted Piperidines Via Ni-Catalyzed β-Carbon Elimination”Org. Lett. 2012, 14, 2026-2029; Synfacts Highlight 2012, 8(7), 0715;
Synfacts Highlight 2012, 8(9), 0949. PubMed PMID: 22468962; PubMed Central PMCID: PMC4138124.
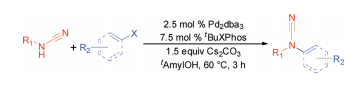
R. M. Stolley, W. X. Guo,† J. Louie “Palladium-Catalyzed Cross-Coupling of Cyanamides”Org. Lett. 2012, 14, 322-325; C & E News December 19, 2011. PubMed PMID: 22142553; PubMed Central PMCID: PMC4113087.
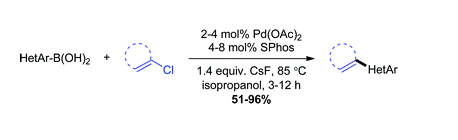
Thakur, K. Zhang, J. Louie “Suzuki-Miyaura Coupling if Hetero-aryl Boronic Acids and Vinyl Chlorides” Chem. Comm. 2012, 48, 203-205.
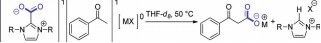
"Imidazolidene Carboxylate Bound MBPh4 Complexes (M=Li, Na) and their Relevance in Transcarboxylation Reactions." Van Ausdall, B.; Poth, N.; Kincaid, V.; Arif, A.; Louie, J. J. Org. Chem. 2011, 76, 8413-8420.
"Nickel-Mediated Cycloadditions by Two Sequential C-H Activations." Kumar, P.; Louie, J. Angew. Chem. Int. Ed. 2011, 50, 10768-10769

"A Serendipitous Discovery: Nickel Catalyst for the Cycloaddition of Diynes w/ Unactivated Nitriles." Kumar, P.; Prescher, S.; Louie, J. Angew. Chem. Int. Ed. 2011, 50, 10694-10698
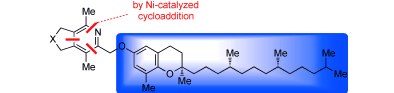
"Nickel-Catalyzed [2+2+2] Cycloaddition of Diynes and Cyanamides." Stolley, R.; Maczka, M.; Louie, J. Eur. J. Org. Chem. 2011, 2011, 3815-3824
"Iron-Catalyzed Cycloaddition of Alkynenitriles and Alkynes." D'Souza, B.; Lane, T.; Louie, J. Org. Lett. 2011, 13, 2936-2939
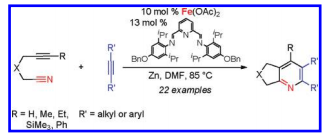
"Ketenes Finally Give in to Cycloadditions." Kumar, P.; Troast, D.; Cella, R.; Louie, J. Chemical Engineering News: Science and Technology Concentrates 2011
"Ni-Catalyzed Ketene Cycloaddition: A System That Resists the Formation of Decarbonylation Side Products." Kumar, P.; Troast, D.; Cella, R.; Louie, J. J. Am. Chem. Soc. 2011, 133, 7719-7721

"Rhodium-Catalyzed Decarboxylative Cycloaddition Route to Substituted Anilines." Zhang, K.; Louie, J. J. Org. Chem., 2011, 76, 4686-4691

"N-Heterocyclic Carbene Bound Nickel(1) Complexes and Their Roles in Catalysis." Zhang, K.; Conda-Sheridan, M.; Cooke, S.; Louie, J. Organometallics, 2011, 30, 2546-2552
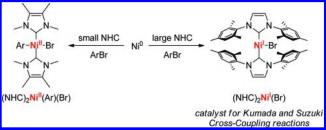
"A Systematic Investigation of Factors Influencing the Decarboxylation of Imidazolium Carboxylates." Van Ausdall, B.; Glass, J.; Wiggins, K.; Aarif, A.; Louie, J. J. Org. Chem., 2009, 74, 7935-7942
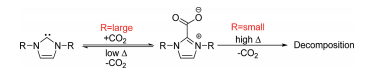
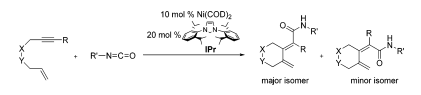
B. R. D’Souza, J. Louie “Nickel Catalyzed Cycloadditive Couplings of Enynes and Isocyanates” Org. Lett. 2009, 11, 4168-4171
S. Wang, D. M. Troast, M. Conda-Sheridan, G. Zuo, D. LaGarde, J. Louie, D. Tantillo “Mechanism of the Ni(0)-Catalyzed Vinylcyclopropane-Cyclopentene Rearrangement” J. Org. Chem. 2009, 74, 7822-7833
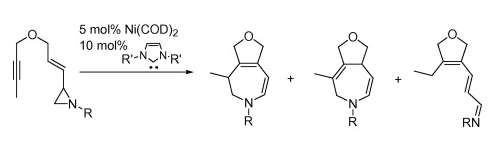
G. Zuo, K. Zhang, J. Louie “Nickel Catalyzed Reactions of Vinyl Aziridines and Aziridinylen-ynes” Tetrahedron Lett. 2008, 49, 6797-6799.
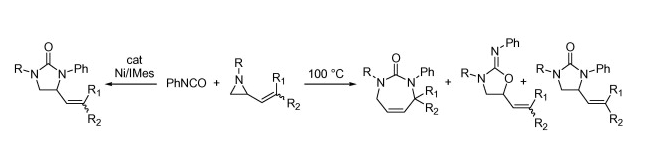
K. Zhang, P. R. Chopade, J. Louie “Coupling of Vinyl Aziridines and Phenyl Isocyanate” Tetrahedron Lett. 2008, 49, 4306-4309.
T. N. Tekavec, J. Louie “Nickel-Catalyzed Cycloisomerization of Enynes: Mechanistic Evidence for Catalyst Generation via C-H Activation of Carbene Ligands” Tetrahedron 2008, 64, 6870-6875; Invited Contribution to a Special Edition Honoring Professor John F. Hartwig’s Young Investigator Prize.
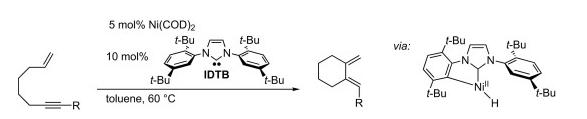
T. N. Tekavec, J. Louie “Nickel-Catalyzed Cycloaddition of Enynes and Carbonyls” J. Org. Chem. 2008, 73, 2641-2648.
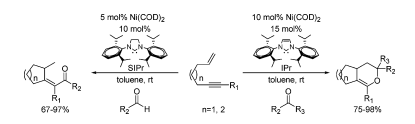
J. Louie “Bis[tri(o-tolyl)phosphine]palladium” in Encyclopedia of Reagents for Organic Synthesis; Paquette, L. A., ed.; John Wiley & Sons Ltd.: Chichester, 2007.
J. Louie, T. N. Tekavec “Transition metal-catalyzed reactions using N-heterocyclic carbene ligands (besides Pd-catalyzed and metathesis reactions)” in N-heterocyclic carbenes in transition metal catalysis; Glorius, F., ed.; Wiley-VCH: Weinheim, 2006.
J. Louie, G. Zuo, H. Duong “Bis(1,5-cyclooctadiene)nickel [update]” in Encyclopedia of Reagents for Organic Synthesis; Paquette, L. A., ed.; John Wiley & Sons Ltd.: Chichester, 2006.
J. Louie “Ni-NHC mediated catalysis” in N-Heterocyclic Carbenes in Synthesis; Nolan, S. P., ed.; Wiley-VCH: Weinheim, 2006.
P. R. Chopade, J. Louie “[2+2+2] Cycloaddition Reactions Catalyzed by Transition Metal Complexes” Adv. Synth. Catal. 2006, 348, 2307-2327.
T. N. Tekavec, G. Zuo, K. Simon,† J. Louie “An In Situ Ni Catalyst for Cycloaddition Reactions” J. Org. Chem. 2006, 71, 5834-5836.
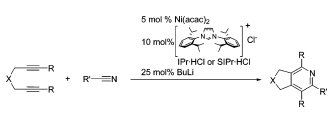
H. A. Duong, J. Louie “A Nickel-Catalyzed Cycloaddition of Alkynes and Isocyanates that Affords Pyrimidine-diones” Tetrahedron 2006, 62, 7547-7551; Invited Contribution to a Special Edition on Recent Advances in Organonickel Chemistry.
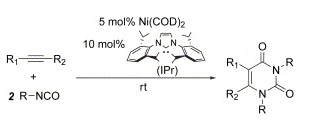
H. A. Duong, J. Louie “Regioselectivity in Ni/Phosphine Catalyzed Cycloadditions of Alkynes and Isocyanates” J. Organomet. Chem. 2005, 690, 5098-5104; Invited Contribution to a Special Issue (Organometallic Chemistry - The Next Generation).
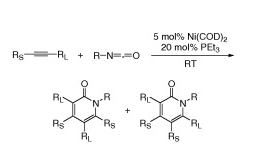
T. N. Tekavec, J. Louie “Nickel-Catalyzed Cycloadditions of Unsaturated Hydrocarbons and Carbonyls Compounds” Org. Lett. 2005, 7, 4037-4039.
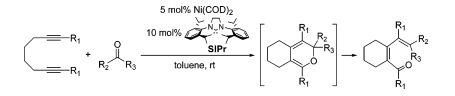
G. Zuo, J. Louie “Selectivity in Nickel-Catalyzed Rearrangements of Cyclopropylen-ynes” J. Am. Chem. Soc. 2005, 127, 5798-5799.
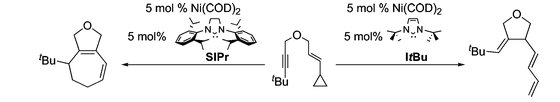
M. M. McCormick, H. A. Duong, G. Zuo, J. Louie “A Nickel-Catalyzed Route to Pyridines” J. Am. Chem. Soc. 2005, 127, 5030-5031.
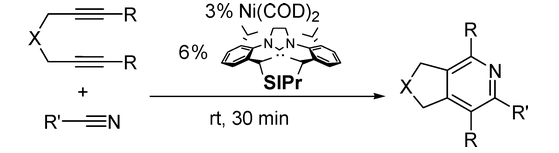
J. Louie “Transition Metal Catalyzed Reactions of Carbon Dioxide and Other
Heterocumulenes” Curr. Org. Chem. 2005, 9, 605-623; Invited Contribution to a Special Issue on Metal-Catalyzed
Reactions.
H. A. Duong, M. J. Cross, J. Louie “N-Heterocyclic Carbenes as Highly Efficient Catalysts for the Cyclotrimerization of Isocyanates” Org. Lett. 2004, 6, 4679-4681.
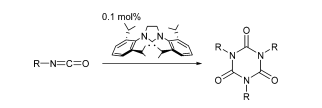
H. A. Duong, M. J. Cross, J. Louie “Nickel-Catalyzed Cycloadditions of Alkynes and Isocyanates” J. Am. Chem. Soc. 2004, 126, 11438-11439.
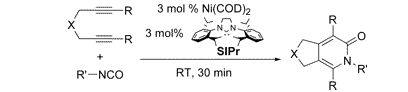
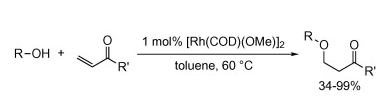
M. V. Farnworth,† M. J. Cross, J. Louie “Rhodium-Catalyzed Addition of Alcohols to Terminal Enones” Tetrahedron Lett. 2004, 45, 7441-7443.
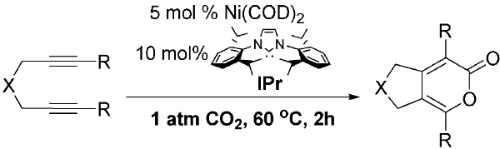
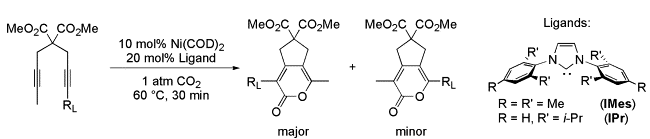
T. N. Tekavec, A. M. Arif, J. Louie “Regioselectivity in Nickel-Catalyzed Cycloadditions of CO2 and Diynes” Tetrahedron 2004, 60, 7431-7437; Invited Contribution to a Special Edition Honoring Professor Robert H. Grubbs’ Tetrahedron Prize.
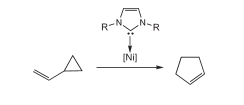
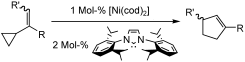
G. Zuo, J. Louie “Highly Active Nickel Catalysts for the Isomerization of Unactivated Vinyl Cyclopropanes to Cyclopentenes” Angew. Chem. Int. Ed. 2004, 43, 2277-2279; “Hot Paper” Web Release 03/24/2004.
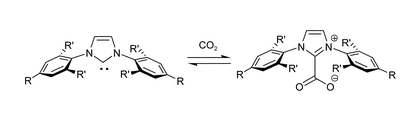
H. A. Duong, T. N. Tekavec, A. M. Arif, J. Louie “Reversible Carboxylations of N-Heterocyclic Carbenes” Chem. Comm. 2004, 1, 112-113.
Louie, J. E. Gibby,† M. V. Farnworth,† T. N. Tekavec ††“”“Efficient Nickel-Catalyzed [2+2+2] Cycloaddition of CO2 and Diynes” J. Am. Chem. Soc. 2002, 124, 15188-15189; 2004, 8590 (addition/correction).