Gary E. Keck
 ORGANIC CHEMISTRY
ORGANIC CHEMISTRY
Distinguished Professor
B.S., Bowling Green State University, 1971
Ph.D., University of Wisconsin, Madison, 1975
Postdoctoral, Harvard University, 1975
Phone: (801) 581-7055
Office: 3274 HEB-N
Email: keck@chem.utah.edu
Activities & Awards
- Alfred P. Sloan Fellow, 1981-85
- Utah Award by the Central Utah and Salt Lake Sections of the American Chemical Society, 2011
- University of Utah Distinguished Scholarly & Creative Research Award, 2012
- Cope Scholar Award, 2014
- Distinguished Professor, U of U, 2014
Research Interests
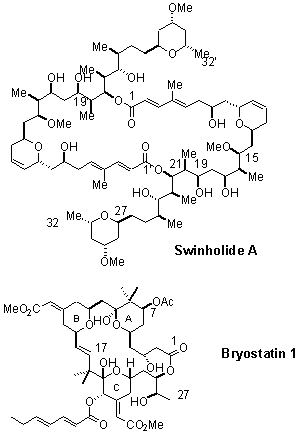
Research in our group focuses heavily on the total synthesis of natural products, particularly those with desirable biological activity of some sort. Representative of the structures currently of interest to us are the cytotoxic macrodiolide swinholide (1), and bryostatin 1 (2), a complex macrolide that is showing exciting activity in clinical trials against a variety of human cancers.
Closely related to our natural products work is our research on new synthetic methods, that is, the design of useful new organic reactions for applications in our total synthesis efforts. Often these evolve in a very natural way, in response to specific problems encountered in a synthesis or in response to certain key structural features presented by a given target.
Much of our current research in this area is focused upon the development of methodology for use in the synthesis of complex materials where stereochemistry is of primary concern. Many of these reaction types involve the complexation and activation of a carbonyl compound by a Lewis acid of some sort, and studies of both complexation phenomena and reaction mechanisms via variable temperature multinuclear NMR spectroscopy have proven valuable in developing and refining such reactions. A current emphasis is on the use of chiral Lewis acid catalysts that we have developed to carry out transformations that yield predominantly one enantiomer of product from achiral reactants. The design of synthetically useful reactions involving radical intermediates represents another long-standing interest in our research group, and these have been applied in several syntheses as well.
Hence, due to our blend of total synthesis with methods development, my students are exposed to, and work in, a wide variety of areas, from theory and reaction mechanisms to conformational analysis, spectroscopy, and computational chemistry. Natural products synthesis is thus, in our view, an excellent vehicle for the development of the intellectual acumen and experimental techniques necessary for success in any area of organic research.
Finally, it should be noted that this type of research is very demanding yet equally rewarding. It is difficult to describe the satisfaction of seeing the new reactions one develops successfully applied to the synthesis of an important natural product, or the excitement at the end of a synthesis as the final spectral and chromatographic comparisons of natural and synthetic material are completed. The strange dichotomy between the infinite number of possible approaches to a given complex structure and the bottom line of such investigations-exact synthetic reconstruction of a given naturally occurring structure-makes such research both intellectually fulfilling and emotionally exciting.
Selected Publications
- “Total Synthesis of Bryostatin 1” Gary E. Keck, Yam B. Poudel, Thomas J. Cummins, Arnab Rudra, and Jonathan A. Covel, J. Am. Chem. Soc.2011, 133, 744-747. click here for the full text
- “The Synthetic Bryostatin Analog Merle 23 Dissects Distinct Mechanisms of Bryostatin Activity in the LNCaP Human Prostate Cancer Cell Line” Noemi Kedei, Gabriella Czifra, Matthew B. Kraft, Anh P. Truong, Wei Li, Carina C. Sanchez, Gary E. Keck, and Peter M. Blumberg, Biochemical Pharmacology, 2011, 81, 1296-1308. click here for the full text
- “Some Phorbol Esters May Partially Resemble Bryostatin 1 in Their Actions on LNCaP Prostate Cancer Cells and U937 Leukemia Cells” Noemi Kedei, Emanuel Lubart, Nancy E. Lewin, Andrea Telek, Langston Lim, Poonam Mannam, Susan H. Garfield, Matthew B. Kraft, Gary E. Keck, Raz Jelinek, and Peter M. Blumberg, ChemBioChem.2011, 12, 1242-1251. click here for the full text
- “Molecular Modeling, Total Synthesis, and Biological Evaluations of C9-Deoxy Bryostatin 1” Gary E. Keck, Yam B. Poudel, Arnab Rudra, Jeffrey C. Stephens, Noemi Kedei, Nancy E. Lewin, Megan L. Peach, and Peter M. Blumberg, Angew. Chemie Int. Ed.2010, 49, 4580–4584. (PMID 20491108) click here for the full text
- “The Bryostatin 1 A-Ring Acetate is Not the Critical Determinant for Antagonism of Phorbol Ester-Induced Biological Responses” Gary E. Keck, Wei Li, Matthew B. Kraft, Noemi Kedei, Nancy E. Lewin, and Peter M. Blumberg, Org. Lett. 2009, 11, 2277-2280. (PMID: 19419164) click here for the full text
- “Substitution on the A Ring Confers to Bryopyran Analogues the Unique Biological Activity Characteristic of Bryostatins and Distinct From That of the Phorbol Esters” Gary E. Keck, Yam B. Poudel, Dennie S. Welch, Matthew B. Kraft, Anh P. Truong, Jeffrey C. Stephens, Noemi Kedei, Nancy E. Lewin, and Peter M. Blumberg, Org. Lett.2009, 11, 593-596. click here for the full text
- “Diastereoselective Synthesis of Cyclopentapyridazinones via Radical Cyclization: Synthetic Studies Toward Halichlorine” Gary E. Keck and Stacey A. Heumann, Org. Lett. 2008, 10, 4783-4786. click here for the full text
- “Total Synthesis of Epothilones B and D: Stannane Equivalents for β-Keto Ester Dianions” Gary E. Keck, Robert L. Giles, Victor J. Cee, Carrie A. Wager, Tao Yu, and Matthew B. Kraft, J. Org. Chem.2008, 73, 9675-9691. (A. I. Meyers Memorial Issue) click here for the full text
- "Convergent Assembly of Highly Potent Analogues of Bryostatin 1 via Pyran Annulation: Bryostatin Look-Alikes that Mimic Phorbol Ester Function" Gary E. Keck, Matthew B. Kraft, Anh P. Truong, Wei Li, Carina C. Sanchez, Noemi Kedei, Nancy Lewin, and Peter M. Blumberg, J. Am. Chem. Soc. 2008, 130, 6660-6661. click here for the full text
- “A New Construction of 2-Alkoxypyrans by an Acylation-Reductive Cyclization Sequence” Lars V. Heumann and Gary E. Keck, Org. Lett. 2007, 9, 1951-1954. click here for the full text
- "Synthetic Studies Toward Bryostatin 1: Preparation of a C1–C16 Fragment by Pyran Annulation", G. E. Keck, D. S. Welch, and Y. B. Poudel, Tetrahedron Lett. 2006, 47, 8267-8270. click here for the full text
- "Synthetic Studies Toward the Bryostatins: A Substrate-Controlled Approach to the A-Ring", G. E. Keck, D. S. Welch, and P. K. Vivian, Org. Lett. 2006, 8, 3667-3670. click here for the full text
- "Total Synthesis of (+)-Dactylolide", C. C. Sanchez and G. E. Keck, Org. Lett. 2005,7, 3053-3056. click here for the full text
- "Synthetic Studies on the Bryostatins: Synthetic Routes to Analogues Containing the Tricyclic Macrolactone Core", G. E. Keck and A.P. Truong, Org. Lett. 2005, 7, 2153-2156. click here for the full text
- “Pyran Annulation: Asymmetric Synthesis of 2,6-Disubstituted-4-methylene Tetrahydropyrans”, Gary E. Keck, Jonathan A. Covel, Tobias Schiff, and Tao Yu, Org. Lett.2002, 4, 1189-1192. click here for the full text
- "Asymmetric Total Synthesis of Rhizoxin D", G. E. Keck, C. A. Wager, T. T. Wager, K. A. Savin, J. A. Covel, M. D. McLaws, D. Krishnamurthy, and V. J. Cee, Angew. Chem. Int. Ed.2001, 40, 231-234. click here for the full text
- "The First Directed Reduction of b-Alkoxy Ketones to Anti-1, 3-Diol Monoethers; Identification of Spectator and Director Alkoxy Groups", G. E. Keck and C. A. Wager, Org. Lett. 2000, 2, 2307-2309. click here for the full text
- "Catalytic Asymmetric Allylation Reactions Using BITIP Catalysis and 2-Substituted Allylstannanes as Surrogates for β-Keto Ester Dianions", G. E. Keck and T. Yu, Org. Lett. 1999, 1, 289-292. click here for the full text
- "A Second-Generation Radical-Based Synthesis of (+)-7-Deoxypancratistatin", G. E. Keck, T. T. Wager, and S. F. McHardy, J. Org. Chem.1998, 63, 9164-9165. click here for the full text
