C. Dale Poulter
 BIOORGANIC CHEMISTRY
BIOORGANIC CHEMISTRY
Distinguished Professor Emeritus
B.S., Louisiana State University, 1964
Ph.D., University of California, Berkeley, 1967
NIH Postdoctoral Fellow, University of California, Los Angeles, 1967
Phone: 801-581-6685
Office: 2274 HEB-N
Email: poulter@chem.utah.edu
Research Group
Biological Chemistry Program
Activities & Awards
- Nakanishi Prize, American Chemical Society
- James Flack Norris Award, American Chemical Society
- Repligen Award, Biological Division, American Chemical Society
- Ernest Guenther Award, American Chemical Society
- Arthur C. Cope Scholar Award, American Chemical Society
- Alfred P. Sloan Fellow
- National Institute of Health Research Career Development Award
- Rosenblatt Prize, University of Utah
- David P. Gardner Fellow
- Distinguished Research Award, University of Utah
- Utah Award, American Chemical Society
- The Governor's Medal for Science and Technology
- Editor-in-Chief, Journal of Organic Chemistry
- Executive Committee, Biological Division of the American Chemical Society
- Fellow, American Academy of Arts and Sciences
- Member, National Academy of Sciences
Research Interests
My research group is interested in problems at the interface between organic chemistry and biochemistry. We study reactions catalyzed by enzymes in the isoprene biosynthetic pathway with special emphasis on establishing the mechanisms of the enzyme-catalyzed transformations and how the enzymes promote the reactions.
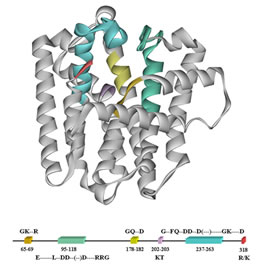
We also study structure-function relationships in peptides and proteins to determine how the particular topology of a complex biological molecule is related to its function as a catalyst or a ligand for receptor binding.
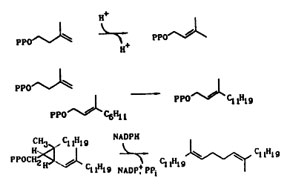
The isoprene biosynthetic pathway is needed by all organisms to produce essential compounds. We are studying several key enzymes in the pathway that catalyze fundamental reactions, including the isomerization of isopentenyl diphosphate (IPP) to dimethylallyl diphosphate (DMAPP), the condensation of IPP with a variety of allylic diphosphates to yield new allylic isoprenylogues containing five additional carbons, the unusual rearrangement of presqualence diphosphate (PSPP) to squalene during biosynthesis of cholesterol, and the posttranslational modifications of proteins by isoprenoid units that are important in cell signalling events. We are also studying unusual isoprenoid alkylations that occur during biosynthesis of ergot alkaloids, post-translational modifications of proteins, and in transfer RNAs.
We are isolating genes for the enzymes we study, construct plasmids for overexpression of the enzymes, and carrying out side-directed mutagenesis on critical amino acids to elucidate their role in catalysis. All of the reactions presented above are interesting biological alkylations in that they do not rely on commonly observed carbonyl group chemistry for construction of carbon-carbon and carbon--heteroatom bonds.Work in this area provides training in a combination of biochemical (purification of enzymes, kinetics, precursor-product studies) and chemical (synthesis, isolation-identification, reaction mechanisms) techniques.
 Much of what we do relies heavily on modern analytical methods such as high pressure
liquid chromatography, high field multinuclear magnetic resonance spectroscopy, and
gas chromatography-mass spectrometry. We draw on ideas and techniques from organic
chemistry and biochemistry to design our experiments, including developing new procedures
for synthesis of compounds, developing new methods for measuring interactions between
an enzyme and its substrates, and using recombinant DNA technology to obtain biological
molecules that are normally difficult to isolate. It is the interdisciplinary nature
of our work that we find both challenging and exciting.
Much of what we do relies heavily on modern analytical methods such as high pressure
liquid chromatography, high field multinuclear magnetic resonance spectroscopy, and
gas chromatography-mass spectrometry. We draw on ideas and techniques from organic
chemistry and biochemistry to design our experiments, including developing new procedures
for synthesis of compounds, developing new methods for measuring interactions between
an enzyme and its substrates, and using recombinant DNA technology to obtain biological
molecules that are normally difficult to isolate. It is the interdisciplinary nature
of our work that we find both challenging and exciting.
Selected Publications
- Ahrens-Botzong, A.; Janthawornpong, K.; Wolny, J.A.; Tambou, E. N.; Rohmer, M.; Krasutsky, S.; Poulter, C. D.; Schunemann, V.; Seemann, M. “Biosynthesis of Isoprene Units: Mossbauer Spectroscopy of Substrate and Inhibitor Binding to the [4Fe-4S] Cluster of the LytB/IspH Enzyme” Angew. Chem. Int. Ed., 2011, 50, 11976-11979.
- Nagai, T.; Unno, H.; Janczak, M.W.; Yoshimura, T.; Poulter, C.D.; Hemmi, H. “Covalent Modification of Reduced Flavin Mononucleotide in Type 2 Isopentenyl Diphosphate Isomerase by Active-Site-Directed Inhibitions” Proc. Natl. Acad. Sci. USA, 2011, 108, 20461-20466.
- Mabanglo, M. F.; Pan, J.-J.; Shakya, B.; Poulter, C. D. “Mutagenesis of isopentenyl phosphate kinase to enhance geranyl phosphate kinase activity” ACS Chem. Biol.2012, 7, 124-1246.
-
Rudolf, J. D.; Wang, H.; Poulter, C. D. “Multisite Prenylation of 4-Substituted Tryptophans by Dimethylallyltryptophan Synthase” J. Am. Chem. Soc.2013, 135, 1895-1902.
- Janthawornpong, K.; Krasutsky, S.; Chaignon, P.; Rohmer, M.; Poulter, C. D.; Seemann, M. “Inhibition of IspH, a [4Fe−4S]2+ Enzyme Involved in the Biosynthesis of Isoprenoids via the Methylerythritol Phosphate Pathway” J. Am. Chem. Soc.2013, 135, 1816-1822.
- Wallrapp, F. H.; Pan, J.-J.; Ramamoorthy, G.; Almonacid, D. E.; Hillerich, B. S.; Seidel, R.; Patskovsky, Y.; Babbitt, P. C.; Almo, S. C.; Jacobson, M. P.; Poulter, C. D. “Prediction of function for the polyprenyl transferase subgroup in the isoprenoid synthase superfamily” Proc. Natl. Acad. Sci.2013, 110, E1196-E1202.
- Seo, J.-S.; Lee, S.; Poulter, C. D. “Regioselective Covalent Immobilization of Recombinant Antibody-Binding Proteins A, G, and L for Construction of Antibody Arrays” J. Am. Chem. Soc.2013, 135, 8973-8980.
- Rudolf, J. D.; Poulter, C. D. “Tyrosine O-Prenyltransferase SirD Catalyzes S-, C-, and N-Prenylations on Tyrosine and Tryptophan Derivatives” ACS Chemical Biology2013, 8, 2707-2714.
- Choi, S.-R..; Seo, J.-S.; Bohaty, R. F. H.; Poulter, C.D. “Regio- and Chemoselective Immobilization of Proteins on Gold Surfaces” Bioconjugate Chem. 2014, 25, 269-275.
- de Ruyck, J.; Janczak, M. W.; Neti, S. S.; Rothman, S. C.; Schubert, H. L; Cornish, R. M.; Matagne, A.; Wouters, J.; Poulter, C. D. “Determination of Kinetics and the Crystal Structure of a Novel Type 2 Isopentenyl Diphosphate: Dimethylallyl Diphosphate Isomerase from Streptococcus pneumonia” ChemBioChem, 2014, 15, 1452-1458.
